Chemical Physics Letters 195, 67-76 (1992)
Tatiana Cwioka, Bogumil Jeziorskia, Wlodzimierz Kolosa,
Robert Moszynskia, J. Rychlewskib, and Krzysztof Szalewiczc
a Department of Chemistry, University of Warsaw, Pasteura 1, 02093 Warsaw, Poland
b Department of Chemistry, A. Mickiewicz University, ul. Grunwaldzka 6, 60-780 Poznan, Poland
c Department of Physics and Astronomy, University of Delaware, Newark, DE 19716, USA
Convergence properties and large-order behavior of the polarization expansion
for the interaction energy of hydrogen atoms
Abstract
High-order corrections in the polarization expansion for the interaction energy of two ground-state hydrogen atoms are
computed for a wide range of interatomic distances R. At large R, the convergence radius
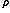 of the expansion is
found to be only slightly greater than unity, e.g.
of the expansion is
found to be only slightly greater than unity, e.g.
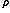 =1.0000000031 at the van der Waals minimum for the triplet state.
The branch points determining
=1.0000000031 at the van der Waals minimum for the triplet state.
The branch points determining
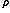 are calculated and used in a convergence acceleration procedure, which shows that the
polarization expansion is a sum of two series - one converging quickly to the Coulomb energy and the other
also converging, although extremely slowly for large R, to the negative of the exchange energy. The total
series converges to the energy of the ground state of the hydrogen molecule. A simple formula describing the large-order
behavior of the polarization series at large R is derived and used to show
that the exchange energy can be accurately calculated from the knowledge of three consecutive large-order polarization energies.
are calculated and used in a convergence acceleration procedure, which shows that the
polarization expansion is a sum of two series - one converging quickly to the Coulomb energy and the other
also converging, although extremely slowly for large R, to the negative of the exchange energy. The total
series converges to the energy of the ground state of the hydrogen molecule. A simple formula describing the large-order
behavior of the polarization series at large R is derived and used to show
that the exchange energy can be accurately calculated from the knowledge of three consecutive large-order polarization energies.
Back
to the publication list of Tatiana Korona
Tatiana Korona
2003-01-17
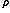 of the expansion is
found to be only slightly greater than unity, e.g.
of the expansion is
found to be only slightly greater than unity, e.g.
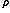 =1.0000000031 at the van der Waals minimum for the triplet state.
The branch points determining
=1.0000000031 at the van der Waals minimum for the triplet state.
The branch points determining
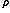 are calculated and used in a convergence acceleration procedure, which shows that the
polarization expansion is a sum of two series - one converging quickly to the Coulomb energy and the other
also converging, although extremely slowly for large R, to the negative of the exchange energy. The total
series converges to the energy of the ground state of the hydrogen molecule. A simple formula describing the large-order
behavior of the polarization series at large R is derived and used to show
that the exchange energy can be accurately calculated from the knowledge of three consecutive large-order polarization energies.
are calculated and used in a convergence acceleration procedure, which shows that the
polarization expansion is a sum of two series - one converging quickly to the Coulomb energy and the other
also converging, although extremely slowly for large R, to the negative of the exchange energy. The total
series converges to the energy of the ground state of the hydrogen molecule. A simple formula describing the large-order
behavior of the polarization series at large R is derived and used to show
that the exchange energy can be accurately calculated from the knowledge of three consecutive large-order polarization energies.