Journal of Chemical Physics 103, 321-332 (1995)
Robert Moszynskia,b,
Tatiana Koronab,
Paul E. S. Wormera, and Ad van der Avoirda
a Institute of Theoretical Chemistry, Nijmegen-SON Research Center, University of Nijmegen, Toernooiveld 1, 6525 ED Nijmegen, The Netherlands
b Department of Chemistry, University of Warsaw, Pasteura 1, 02-093 Warsaw, Poland
Ab initio potential energy surface, infrared spectrum, and second virial coefficient of the He-CO complex
Abstract
Symmetry-adapted perturbation theory has been applied to compute the intermolecular potential energy
surface of the He-CO complex. The interaction energy is found to be dominated by the first-order exchange
contribution and the dispersion energy. The ab initio potential has a single
minimum of 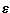 m=-24.895 cm-1
for the linear CO-He geometry at Rm=6.85 bohr.
The computed potential energy surface has been analytically fitted and
used in converged variational calculations to generate bound rovibrational
states of the He-CO molecule and the infrared spectrum, which corresponds
to the simultaneous excitation of vibration and internal rotation in the CO
subunit within the complex. The predicted positions and intensities of lines
in the infrared spectrum are in good agreement with the experimental
spectrum [C.E. Chuaqui et al., J. Chem. Phys. 101, 39 (1994)]. The
theoretical potential was also checked by comparison of computed excess
second virial coefficients with the experimental data. The ab initio interaction
virial coefficients, including quantum corrections, lie within the
experimental error bars over a wide range of temperatures.
m=-24.895 cm-1
for the linear CO-He geometry at Rm=6.85 bohr.
The computed potential energy surface has been analytically fitted and
used in converged variational calculations to generate bound rovibrational
states of the He-CO molecule and the infrared spectrum, which corresponds
to the simultaneous excitation of vibration and internal rotation in the CO
subunit within the complex. The predicted positions and intensities of lines
in the infrared spectrum are in good agreement with the experimental
spectrum [C.E. Chuaqui et al., J. Chem. Phys. 101, 39 (1994)]. The
theoretical potential was also checked by comparison of computed excess
second virial coefficients with the experimental data. The ab initio interaction
virial coefficients, including quantum corrections, lie within the
experimental error bars over a wide range of temperatures.
Back to the publication list of
Tatiana Korona
Tatiana Korona 2003-01-17
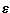 m=-24.895 cm-1
for the linear CO-He geometry at Rm=6.85 bohr.
The computed potential energy surface has been analytically fitted and
used in converged variational calculations to generate bound rovibrational
states of the He-CO molecule and the infrared spectrum, which corresponds
to the simultaneous excitation of vibration and internal rotation in the CO
subunit within the complex. The predicted positions and intensities of lines
in the infrared spectrum are in good agreement with the experimental
spectrum [C.E. Chuaqui et al., J. Chem. Phys. 101, 39 (1994)]. The
theoretical potential was also checked by comparison of computed excess
second virial coefficients with the experimental data. The ab initio interaction
virial coefficients, including quantum corrections, lie within the
experimental error bars over a wide range of temperatures.
m=-24.895 cm-1
for the linear CO-He geometry at Rm=6.85 bohr.
The computed potential energy surface has been analytically fitted and
used in converged variational calculations to generate bound rovibrational
states of the He-CO molecule and the infrared spectrum, which corresponds
to the simultaneous excitation of vibration and internal rotation in the CO
subunit within the complex. The predicted positions and intensities of lines
in the infrared spectrum are in good agreement with the experimental
spectrum [C.E. Chuaqui et al., J. Chem. Phys. 101, 39 (1994)]. The
theoretical potential was also checked by comparison of computed excess
second virial coefficients with the experimental data. The ab initio interaction
virial coefficients, including quantum corrections, lie within the
experimental error bars over a wide range of temperatures.